
2 in 3 Doctors^
Chose wheeze in their top 3 physiological parameters for remote monitoring of adult patients between visits
^In research undertaken with Australian General Practitioners, research conducted by IMC Consulting for Respiri Limited, 2022, n=78
Our solution can help transform the way physicians interact with patients experiencing wheeze while they are away from the clinic. wheezo®, our FDA 510(k) cleared medical device, the respiri™ app (patient user interface) and our health portal can help different health organisations and providers connect with patients to improve collaboration across the care setting.
Reimbursement codes make Remote Patient Monitoring (RPM) a viable new source of revenue for your practice. Ask about wheeze detection programs with RPM.
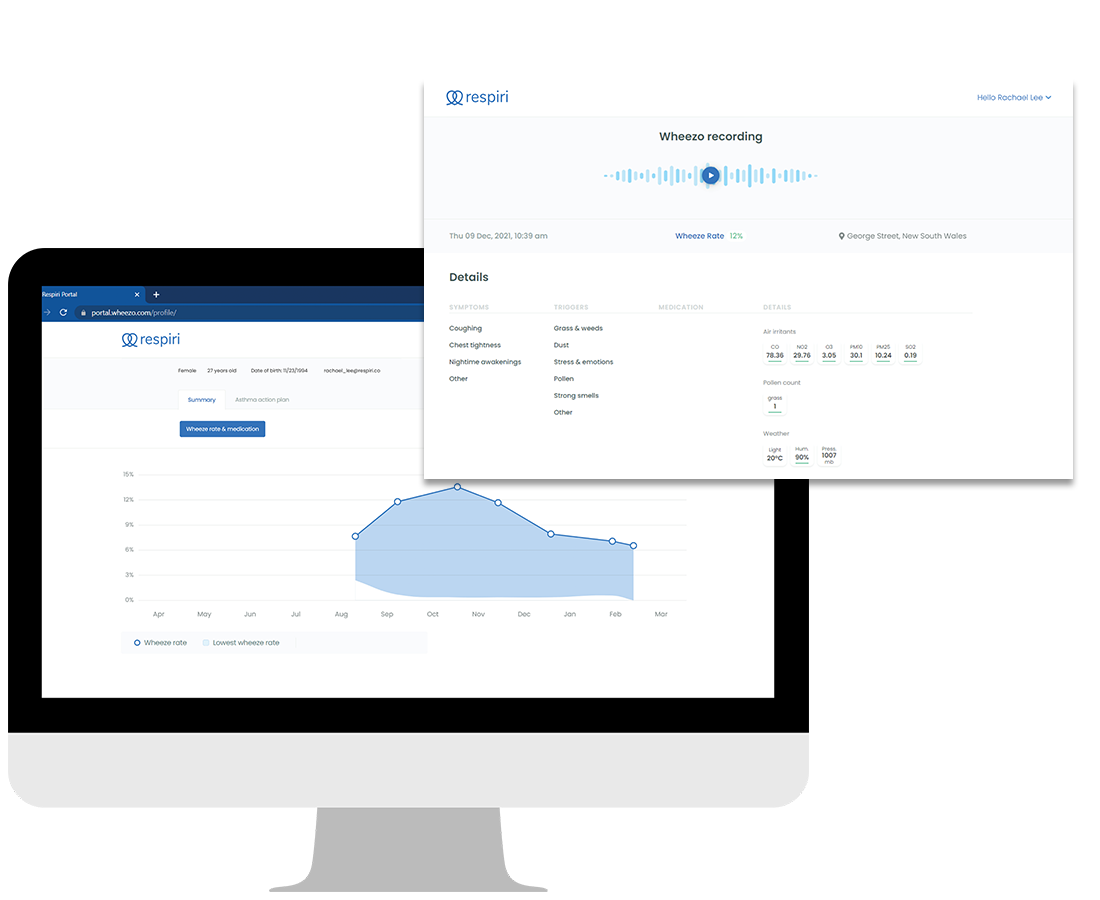
- Wheeze is detected and the system provides a WheezeRate for each breath recording
- WheezeRate history can be viewed longitudinally for each patient via the Respiri health portal
- Wheeze recordings are available for clinician review and immediate playback

Class II medical device
- Integrates into Remote Patient Monitoring (RPM) programs
- breath sensor is non-invasive and easy to operate with no clinical training required
- History of WheezeRates and high-quality playback of wheeze recordings available immediately for clinician review
wheezo® is intended to detect and record abnormal breath sounds (continuous adventitious breath sounds or CABS) at the windpipe (trachea), reported as WheezeRate in adults & children ≥2 years. It is not for diagnostic use. A licensed health care professional’s advice is required to understand the meaning and importance of the wheezo® readings.
Breath sensor
FDA Cleared (510k, K202062)
Class II medical device
Records Breath Sounds
Patient-user interface
Algorithm detects abnormal breath sounds & reports WheezeRate in app
• Self-reported symptoms & triggers
• Real-time environmental data
& Clinical Staff
• Access patient data
• Exceptions based reporting
• Patient engagement & support
• APIs into partner systems
ACOs, HMOs, Hospitals, Private Practice, other
Seamless Integration with
EMR/EHR (i.e. EPIC)
Breath sensor
FDA Cleared (510k, K202062)
Class II medical device
Records Breath Sounds
Patient-user interface
Algorithm detects abnormal breath sounds & reports WheezeRate in app• Self-reported symptoms & triggers
• Real-time environmental data
& Clinical Staff
• Access patient data
• Exceptions based reporting
• Patient engagement & support
• APIs into partner systems
ACOs, HMOs, Hospitals, Private Practice, other
Seamless Integration with
EMR/EHR (i.e. EPIC)
We can integrate our innovative wheeze detection technology with established RPM platforms and programs. Respiri will support you to deliver high-quality care to patients whilst minimizing the administrative demands. Ask us about how we can tailor our solution to meet your needs.
Respiri is proud to work with distribution and marketing partners in the US to offer wheezo®.
Full-service RPM & CCM programs using the Remotli Virtual Care Platform & clinical staff. All the components you need to build a successful connected care program. Choose from a full-service outsourced solution or let Access help you build an internal program. Customized solutions that are just right for your organization.

Are you a Provider or Health Plan interested in a demonstration of our solution? We would love to hear from you.
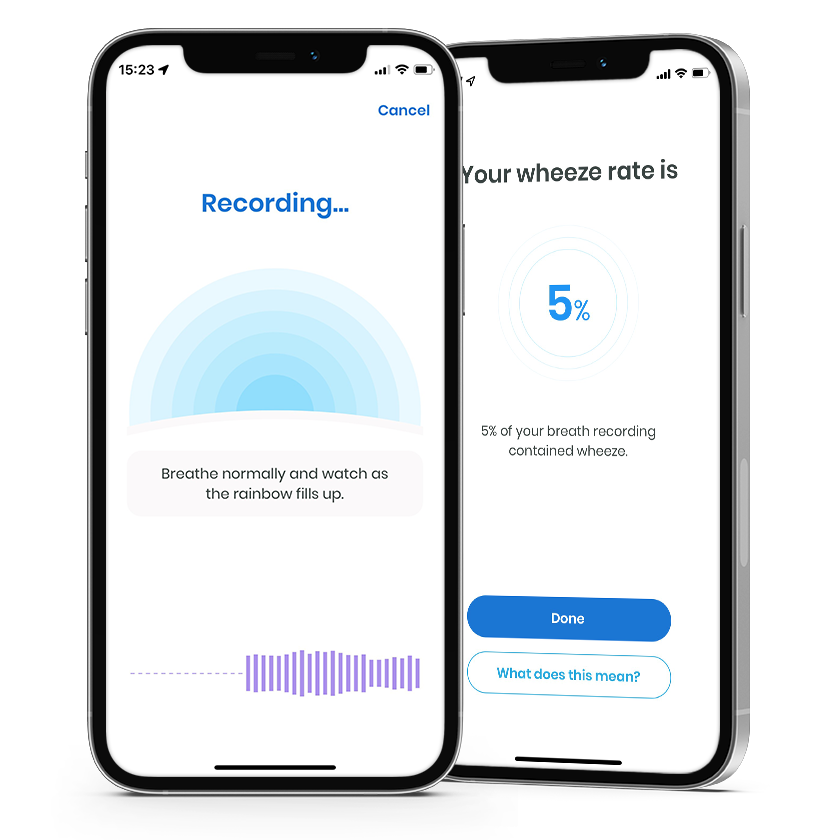
Thanks to wheezo® patients can take breath recordings from home easily, helping medical professionals hear and detect wheeze like never before.
- Connect the device to the respiri™ app via Bluetooth®
- Place device on windpipe to record breath sounds
- Relax and breathe evenly for 30-60 seconds
The respiri™ mobile app runs a wheeze detection algorithm to identify wheeze and display a wheeze rate.
A licensed health care professional’s advice is required to understand the meaning and importance of the wheezo® readings.


Records breath sounds
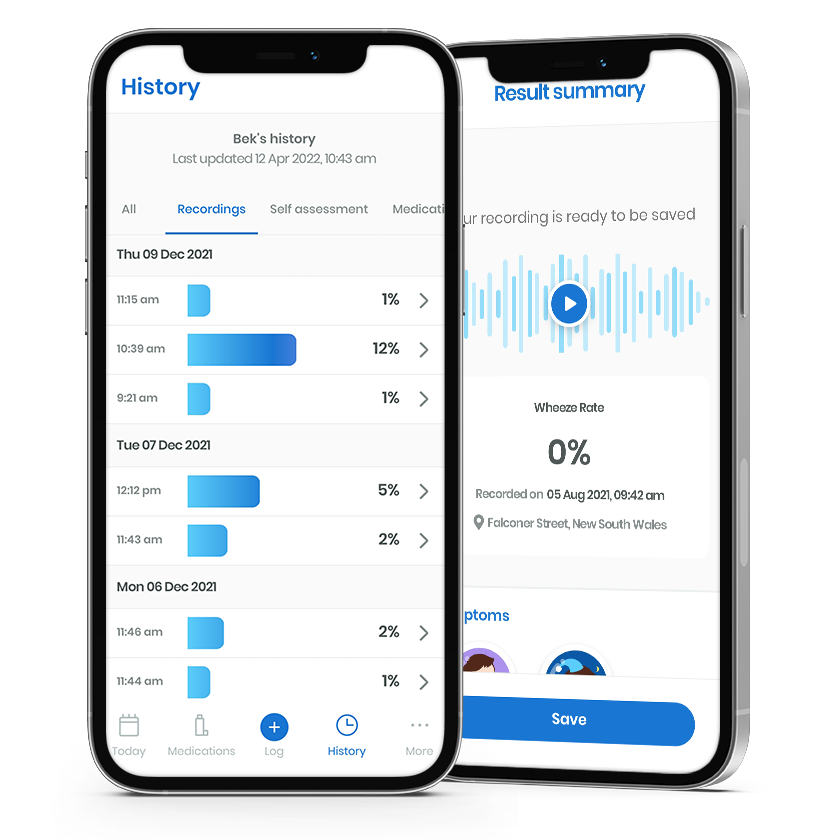
Records WheezeRate history along with breath sound recordings for high-quality playback available in app and via web portal

Records breath sounds


Records WheezeRate history along with breath sound recordings for high-quality playback available in app and via web portal
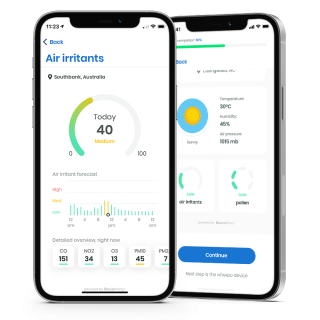
In addition, the respiri™ app also supports patients to log and track self-reported symptoms and triggers. Recordings are stored with weather and environmental data like pollen counts and air irritants present at the time of recording.
Patients can build a personal profile to share WheezeRate history, breath sound recordings and self-reported data with their physicians.
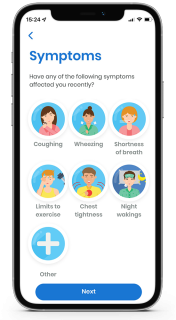
Allows patients to keep a record of self-reported symptoms and triggers
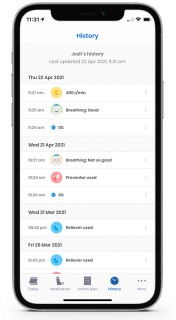
Provides patients and physicians with their complete data history including WheezeRates over time

Patient
Benefits
- Empower your patients. With more data physicians can work with patients helping them gain a better understanding of their wheeze.
- Set medication reminders to encourage better compliance.
- See pollen & air irritant information stored at the time of recordings to see if these may be affecting WheezeRate.

Provider
Benefits
- More data than ever before; to assess and review a patient’s wheeze outside clinic visits.
- Longitudinal data around self-reported symptoms, triggers, and medication use to paint a detailed picture of symptomology.
- A new source of revenue.

Health Plan
Benefits
- Customize care, Respiri, in partnership with its local distributors, will work with you and your providers to develop health programs.
- Real-time analytics, delivering tangible ROI metrics.

- Andrès E, et al. Respiratory sound analysis in the era of evidence-based medicine and the world of medicine 2.0. J Med Life. 2018;11(2):89-106.,Respiratory sound analysis in the era of evidence-based medicine and the world of medicine 2.0
- Dr. M.L. Levy, S. Godfrey, C. S. Irving, A. Sheikh, W. Hanekom, Ambulatory Care Nurses, A. Bush & P. Lachman, ‘Wheeze Detection: Recordings vs. Assessment of Physician and Parent’ (2004) Journal of Asthma, 41:8, 845-853, DOI: 10.1081/JAS-200038451.
- Patel PH, Mirabile VS, Sharma S. ‘Wheezing’ (2021). Available from: https://www.ncbi.nlm.nih.gov/books/NBK482454/
- Cane RS, Ranganathan SC, McKenzie SA, What do parents of wheezy children understand by “wheeze”? Archives of Disease in Childhood 2000;82:327-332.
wheeze
The algorithm analyses the breath recording spectrogram to detects wheeze as well as a respiratory specialist2
A database of 189 recordings was created from 56 patients aged 21-87 years old. Three experts were used; two respiratory physicians and one respiratory technology expert.
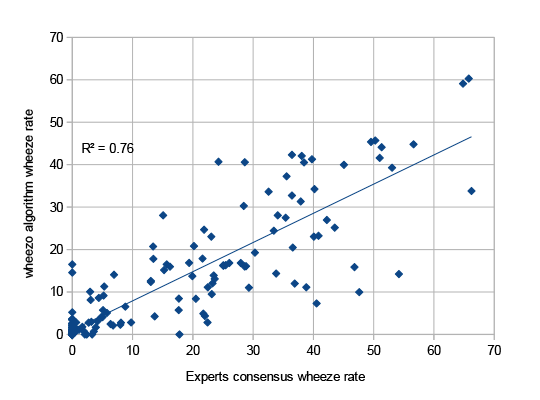
The x-axis is the wheeze rate calculated using the experts consensus and the y-axis is the wheeze rate calculated by the wheezo® algorithm. The value of R squared represents the percent of variation shared between the two variables.
0.81
It’s how patients can detect wheeze
Your physician may have suggested that you use wheezo® together with the respiri™ app to record and detect wheeze. In partnership with your physician, you will create a history of wheeze, store wheeze recordings and log self-reported information about symptoms and possible triggers. This data can be shared with healthcare professionals to give them a clearer picture of your wheeze.
Thanks to wheezo® patients can take breath recordings from home easily, helping healthcare professionals hear wheeze like never before.
patient information
Download the wheezo® Quick Start Guide and Owner’s Manual for further information on how to set up and use your wheezo® breath sensor with the respiriTM app
Instructions for Use / Owner’s Manual
If you have been initiated by your healthcare provider, you can access the full Owner’s Manual (Instructions for Use) from within the respiriTM app. Or contact us using the Customer Support Enquiry form below.






